Why Does an Atom Have No Net Electric Charge
- Its subatomic particles carry no electrical charge. Why does an atom have no net electric charge.
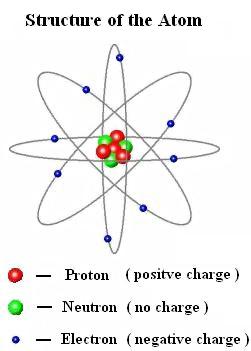
Discoverhover Curriculum Guide 15 Electric Charges And Forces
A net electric charge is caused when the number of electrons out numbers the number of protons within an atom.

. CThe positively charged neutrons cancel out the negatively charged electrons. Due to the fact that electrons can transfer from one atom to another every atom has the possibility of becoming negatively or positively charged. A down quark carries -13 of a q so added together they make 33 or 1 q which is the charge of.
If an atom loses an electron then it has more protons which makes it positively charged. Atoms with net charge are called ions. A neutral atom is one that lacks an electric charge due to a compensation between the number of its protons and electrons that is they have the s ame number of protons and electrons since the total of positive charges of the protons cancels the total of charges.
Atoms with differing charges can bind into what is known as an ion What net charge. BThe positively charged protons cancel out the negatively charged neutrons. For example carbon has 6 protons we know this because the atomic number lower number on the periodic table for carbon is 6.
Why does an atom have no net electric charge. Finally the correct answer is option D. This is the lowest possible energy level of the atom or so called the ground state.
The atoms in their elemental form do not have an effective charge because their particle charges are in balance. DThe positively charged protons cancel out the negatively charged electrons. Tine positively charged neutrons cancel out the negatively charged electrons.
The positively charged protons cancel out the negatively charged electrons How many protons neutrons and electrons does 12652Te have. An atom has no overall charge because each element has the same number of protons and electrons. An atom becomes negatively charged if it gains extra electrons and it becomes positively charged if it loses electrons.
Why does an atom have no net electric charge. Find step-by-step Chemistry solutions and your answer to the following textbook question. Protons are positive neutron are neutral having no charge and electrons are negative.
It is electrically neutral. The positively charged neutrons cancel out the negatively charged electrons. An electron cannot be positioned at the location of a proton at any single point in time without displacing the proton.
Its subatomic particles carry no electric charges. The positively charged protons cancel out the negatively charged neutrons. In an ordinary atom the number of protons equals the number of electrons so the atom normally has no net electric charge.
- Atoms are electrically neutral because they contain equal quantities of positively charged protons and negatively charged electrons. Why does an atom have no net electric charge. A particle with charge cannot exist at the same position and time as another.
It is said that atoms with the same number of electrons as protons are electrically neutral so they have no net charge or net electric field. The positively charged protons cancel out the negatively charged electrons. Jby SHOW ANSWER An atom consists of a positively charged nucleus surrounded by one or more negatively charged particles called electrons.
A proton is made of 2 up quarks and a down quark. 33K views View upvotes Siddhartha Shankar. An up quark carries 23 of a q 16021892 x10-19 Coulomb so two of them makes 43 of a q.
In other words they have a net electric charge equal to zero. AIts subatomic particles carry no electrical charge. Only when an electron is pulled out of an atomic orbital or when one is added do we form ions that have net charges.
Generally the atoms have neutral charge which means they have the same number of electrons and protons. Protons have a 1 charge and electrons have a -1 charge these charges cancel out if there is the same amount of each. 1 Protons and neutrons have internal structure and are made of quarks which carry fractional charge.
The positive charges equal the negative charges so the atom has no overall charge.

Atoms Definition Overview Expii
What Is A Charged Atom Called Quora

Question Video Explaining Why Atoms Do Not Have A Charge Nagwa

Comments
Post a Comment